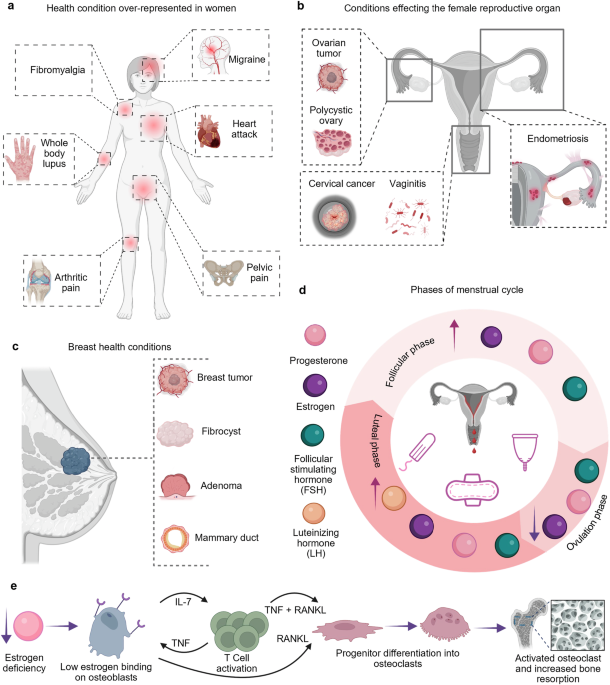
Advances in biomonitoring technologies for women’s health
Shaw, L. J. et al. Quality and equitable health care gaps for women: attributions to sex differences in cardiovascular medicine. J. Am. College Cardiol. 70, 373–388 (2017).
Benyamini, Y. & Todorova, I. Women’s Reproductive Health in Sociocultural Context. Int. J. Behav. Med. 24, 799–802 (2017).
Sutton, M. Y., Anachebe, N. F., Lee, R. & Skanes, H. Racial and ethnic disparities in reproductive health services and outcomes, 2020. Obstet. Gynecol. 137, 225–233 (2021).
Women’s health got worse in 2021, global survey finds. (2022).
Samson Enitan, S. Improving women’s health in the 21st century: current challenges, medical advancements and future prospects. Women Health Care Issues 6, 01–07 (2023).
Google Scholar
Magnus, M. et al. Development of a telehealth intervention to promote care-seeking among transgender women of color in Washington, DC. Public Health Nurs. 37, 262–271 (2020).
Google Scholar
White, J. & Clayton, J. The gender health innovation gap: a perspective from the NIH Office of Research on Women’s Health. Med. 3, 298–301 (2022).
Merone, L., Tsey, K., Russell, D. & Nagle, C. Sex inequalities in medical research: a systematic scoping review of the literature. Women’s Health Rep. 3, 49–59 (2022).
Google Scholar
Lyzwinski, L., Elgendi, M. & Menon, C. Innovative approaches to menstruation and fertility tracking using wearable reproductive health technology: systematic review. J. Med. Internet Res. 26, e45139 (2024).
Zuo, X., Yang, X., Dou, Z. & Wen, J. R. RUCIR at TREC 2019: conversational assistance track. In 28th Text REtrieval Conference, TREC 2019 – Proceedings (National Institute of Standards and Technology (NIST), 2019).
Common chronic diseases in women compared to men. (2021).
WHO. Ten top issues for women’s health. (2015).
Singh, V. AI, women’s health care, and trust: problems and prospects. Artif. Intell. Mach. Learn. Women’s Health Issues 235–254 (2024).
Pan, M. et al. Effects of wearable physical activity tracking for breast cancer survivors: a systematic review and meta-analysis. Int J. Nurs. Knowl. 35, 117–129 (2024).
Google Scholar
Dhingra, L. S. et al. Use of wearable devices in individuals with or at risk for cardiovascular disease in the US, 2019 to 2020. JAMA Netw. Open 6, e2316634 (2023).
Google Scholar
Mattison, G. et al. The influence of wearables on health care outcomes in chronic disease: systematic review. J. Med Internet Res. 24, e36690 (2022).
Google Scholar
Leung, A., Sakkas, D., Pang, S., Thornton, K. & Resetkova, N. Assisted reproductive technology outcomes in female-to-male transgender patients compared with cisgender patients: a new frontier in reproductive medicine. Fertil. Steril. 112, 858–865 (2019).
Google Scholar
Togunwa, T. O., Babatunde, A. O. & Abdullah, K.-R. Deep hybrid model for maternal health risk classification in pregnancy: synergy of ANN and random forest. Front. Artif. Intell. 6, 1213436 (2023).
World Health Organization. More than a third of women experience lasting health problems after childbirth, new research shows. World Health Organization (2023).
World Health Organization. Maternal mortality. World Health Organization (2025).
Goodale, B. M. et al. Wearable sensors reveal menses-driven changes in physiology and enable prediction of the fertile window: observational study. J. Med Internet Res. 21, e13404 (2019).
Google Scholar
Shilaih, M. et al. Modern fertility awareness methods: wrist wearables capture the changes in temperature associated with the menstrual cycle. Biosci. Rep. 38, BSR20171279 (2018).
Vanmarkenlichtenbelt, W. et al. Evaluation of wireless determination of skin temperature using iButtons. Physiol. Behav. 88, 489–497 (2006).
Google Scholar
Garcia, A. M. C. et al. Luteal phase of the menstrual cycle increases sweating rate during exercise. Braz. J. Med. Biol. Res. 39, 1255–1261 (2006).
Google Scholar
Upton, T. J. et al. High-resolution daily profiles of tissue adrenal steroids by portable automated collection. Sci. Transl. Med. 15, eadg8464 (2023).
Google Scholar
myHealth Alberta. Basal Body Temperature (BBT) Tracking. Government of Alberta (2024).
Steward, K. & Raja, A. Physiology, Ovulation and Basal Body Temperature. (2023).
Williams, A. M. FAM basics: basal body temperature (BBT). Nat. Womanhood (2021).
Wei, L. et al. Wearable sweat management technologies. Adv. Mater Technol. 9, 2470031 (2024).
Charkoudian, N. & Stachenfeld, N. Sex hormone effects on autonomic mechanisms of thermoregulation in humans. Auton. Neurosci. 196, 75–80 (2016).
Google Scholar
Baker, F. C., Siboza, F. & Fuller, A. Temperature regulation in women: effects of the menstrual cycle. Temperature 7, 226–262 (2020).
Google Scholar
Zhang, Z., DiVittorio, J. R., Joseph, A. M. & Correa, S. M. The effects of estrogens on neural circuits that control temperature. Endocrinology 162, bqab087 (2021).
Google Scholar
Forman, R. G., Chapman, M. C. & Steptoe, P. C. The effect of endogenous progesterone on basal body temperature in stimulated ovarian cycles. Hum. Reprod. 2, 631–634 (1987).
Google Scholar
Evans-Hoeker, E. et al. Cervical mucus monitoring prevalence and associated fecundability in women trying to conceive. Fertil. Steril. 100, 1033–1038.e1 (2013).
Google Scholar
Boyd, P. et al. A temperature-monitoring vaginal ring for measuring adherence. PLoS One 10, e0125682 (2015).
Google Scholar
Papaioannou, S., Aslam, M., Al Wattar, B. H., Milnes, R. C. & Knowles, T. G. User’s acceptability of OvuSense: a novel vaginal temperature sensor for prediction of the fertile period. J. Obstet. Gynaecol. (Lahore) 33, 705–709 (2013).
Google Scholar
Keeler Bruce, L., González, D., Dasgupta, S. & Smarr, B. L. Biometrics of complete human pregnancy recorded by wearable devices. NPJ Digit Med. 7, 207 (2024).
Google Scholar
Tarvonen, M. et al. Intrapartum cardiotocography with simultaneous maternal heart rate registration improves neonatal outcome. Am. J. Obstet. Gynecol. 230, 379.e1–379.e12 (2024).
Google Scholar
Afari, H. A., Davis, E. F. & Sarma, A. A. Echocardiography for the pregnant heart. Curr. Treat. Options Cardiovasc. Med. 23, 55 (2021).
Google Scholar
Rosen, H. & Yogev, Y. Assessment of uterine contractions in labor and delivery. Am. J. Obstet. Gynecol. 228, S1209–S1221 (2023).
Google Scholar
Leeners, B. Utilizing wearable biosensor technology for monitoring sleep duration patterns in pregnancy – a pilot study. Endocrinol. Disord. 8, 01–10 (2024).
Google Scholar
Ryu, D. et al. Comprehensive pregnancy monitoring with a network of wireless, soft, and flexible sensors in high-and low-resource health settings. Proc. Natl. Acad. Sci. USA 118, e2100466118 (2021).
Google Scholar
Aggarwal, G. & Wei, Y. Non-invasive fetal electrocardiogram monitoring techniques: potential and future research opportunities in smart textiles. Signals 2, 392–412 (2021).
Google Scholar
Mongan, W. et al. A multi-disciplinary framework for continuous biomedical monitoring using low-power passive RFID-based wireless wearable sensors. 2016 IEEE International Conference on Smart Computing, SMARTCOMP 2016 (2016).
Phillips, N. E. et al. The metabolic and circadian signatures of gestational diabetes in the postpartum period characterised using multiple wearable devices. Diabetologia 68, 419–432 (2025).
Google Scholar
Wei, H. X., Yang, Y. L., Luo, T. Y. & Chen, W. Q. Effectiveness of mobile health interventions for pregnant women with gestational diabetes mellitus: a systematic review and meta-analysis. J. Obstet. Gynaecol. (Lahore) 43, 2245906 (2023).
Kytö, M. et al. Behavior change app for self-management of gestational diabetes: design and evaluation of desirable features. JMIR Hum. Factors 9, e36987 (2022).
Google Scholar
Cheung, N. W. et al. A pilot randomised controlled trial of a text messaging intervention with customisation using linked data from wireless wearable activity monitors to improve risk factors following gestational diabetes. Nutrients 11, 590 (2019).
Google Scholar
Kytö, M. et al. Comprehensive self-tracking of blood glucose and lifestyle with a mobile application in the management of gestational diabetes: a study protocol for a randomised controlled trial (eMOM GDM study). BMJ Open 12, e066292 (2022).
Google Scholar
Kytö, M. et al. Supporting the management of gestational diabetes mellitus with comprehensive self-tracking: mixed methods study of wearable sensors. JMIR Diabetes 8, e43979 (2023).
Google Scholar
Penders, J., Altini, M., Van Hoof, C. & Dy, E. Wearable sensors for healthier pregnancies. Proc. IEEE 103, 179–191 (2015).
Google Scholar
Jin, Y. et al. Enhanced detection of Cystatin C for predicting adverse outcomes in gestational diabetes mellitus using a point-of-care immunosensor. Bioelectrochemistry 163, 108907 (2025).
Google Scholar
Ehrlich, S. F. et al. Using a consumer-based wearable activity tracker for physical activity goal setting and measuring steps in pregnant women with gestational diabetes mellitus: exploring acceptance and validity. BMC Pregnancy Childbirth 21, 420 (2021).
Google Scholar
Karnain Wadoo, O., Ahmad, I. & Sayeed, S. I. Reduced lung function and progression to prediabetes: a prospective study. Iran. J. Diabetes Obes. (2021).
Tehrani, F. et al. An integrated wearable microneedle array for the continuous monitoring of multiple biomarkers in interstitial fluid. Nat. Biomed. Eng. 6, 1214–1224 (2022).
Google Scholar
Teymourian, H. et al. Microneedle-based detection of ketone bodies along with glucose and lactate: toward real-time continuous interstitial fluid monitoring of diabetic ketosis and ketoacidosis. Anal. Chem. 92, 2291–2300 (2020).
Google Scholar
Chen, Y. et al. A gold nanoparticles and mxene nanocomposite based electrochemical sensor for point-of-care monitoring of serum biomarkers. ACS Nano 19, 16980–16994 (2025).
Google Scholar
Smyth, S., Curtin, E., Tully, E., Molphy, Z. & Breathnach, F. Smartphone apps for surveillance of gestational diabetes: scoping review. JMIR Diabetes 7, e38910 (2022).
Google Scholar
Asadollahi, F., Zagami, S. E., Eslami, S. & Roudsari, R. L. Barriers and facilitators for mHealth utilization in pregnancy care: a qualitative analysis of pregnant women and stakeholder’s perspectives. BMC Pregnancy Childbirth 25, 141 (2025).
Google Scholar
Walter, J. R. et al. The future of remote monitoring for pregnancy. Bridge (Wash. D. C.) 52, 16 (2022).
Google Scholar
Veinot, T. C., Mitchell, H. & Ancker, J. S. Good intentions are not enough: how informatics interventions can worsen inequality. J. Am. Med. Inform. Assoc. 25, 1080–1088 (2018).
Google Scholar
Girmay, M. Digital health divide: opportunities for reducing health disparities and promoting equitable care for maternal and child health populations. Int. J. Matern. Child Health AIDS 13, e026 (2024).
Google Scholar
Avila-Varela, D. S. et al. Whole-brain dynamics across the menstrual cycle: the role of hormonal fluctuations and age in healthy women. npj Women’s Health 2, 8 (2024).
Google Scholar
Kumar, P. & Sait, S. Luteinizing hormone and its dilemma in ovulation induction. J. Hum. Reprod. Sci. 4, 2–7 (2011).
Lee, Y. & Gao, W. Non-invasive hormone monitoring with a wearable sweat biosensor. Nat. Rev. Bioeng. (2025).
Ye, C. et al. A wearable aptamer nanobiosensor for non-invasive female hormone monitoring. Nat. Nanotechnol. 19, 330–337 (2023).
Google Scholar
Altindag, O. et al. Relation of cortisol levels and bone mineral density among premenopausal women with major depression. Int. J. Clin. Pract. 61, 416–420 (2007).
Google Scholar
Qiao, L., Benzigar, M. R., Subramony, J. A., Lovell, N. H. & Liu, G. Advances in sweat wearables: sample extraction, real-time biosensing, and flexible platforms. ACS Appl Mater. Interfaces 12, 34337–34361 (2020).
Google Scholar
Lee, H. B., Meeseepong, M., Trung, T. Q., Kim, B. Y. & Lee, N. E. A wearable lab-on-a-patch platform with stretchable nanostructured biosensor for non-invasive immunodetection of biomarker in sweat. Biosens. Bioelectron. 156, 112133 (2020).
Google Scholar
Li, Z., Chen, F., Zhu, N., Zhang, L. & Xie, Z. Tip-enhanced sub-femtomolar steroid immunosensing via micropyramidal flexible conducting polymer electrodes for at-home monitoring of salivary sex hormones. ACS Nano 17, 21935–21946 (2023).
Google Scholar
Sekar, M., Pandiaraj, M., Bhansali, S., Ponpandian, N. & Viswanathan, C. Carbon fiber based electrochemical sensor for sweat cortisol measurement. Sci. Rep. 9, 1–14 (2019).
Google Scholar
Zheng, J., Feng, Q., Zheng, S. & Xiao, X. Maternal nutrition and the developmental origins of osteoporosis in offspring: Potential mechanisms and clinical implications. Exp. Biol. Med. 243, 836–842 (2018).
Google Scholar
Madigan, J. A. et al. Perinatal hair cortisol concentrations linked to psychological distress and unpredicted birth complications. Psychoneuroendocrinology 161, 106921 (2024).
Google Scholar
Stirrat, L. I. et al. Pulsatility of glucocorticoid hormones in pregnancy: Changes with gestation and obesity. Clin. Endocrinol. (Oxf.) 88, 592–600 (2018).
Google Scholar
Hassan, S., Muere, A. & Einstein, G. Ovarian hormones and chronic pain: a comprehensive review. Pain 155, 2448–2460 (2014).
Google Scholar
Chantalat, E. et al. Estrogen receptors and endometriosis. Int. J. Mol. Sci. 21, 2815 (2020).
Google Scholar
Patel, S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J. Steroid Biochem Mol. Biol. 182, 27–36 (2018).
Google Scholar
Hussain, S. M., Cicuttini, F. M., Alyousef, B. & Wang, Y. Female hormonal factors and osteoarthritis of the knee, hip and hand: a narrative review. Climacteric 21, 132–139 (2018).
Google Scholar
Pérez-López, F. R., Larrad-Mur, L., Kallen, A., Chedraui, P. & Taylor, H. S. Gender differences in cardiovascular disease: hormonal and biochemical influences. Reprod. Sci. 17, 511–531 (2010).
Google Scholar
Chakraborty, S., Ganti, A. K., Marr, A. & Batra, S. K. Lung cancer in women: role of estrogens. Expert Rev. Respir. Med. 4, 509–518 (2010).
Google Scholar
Meng, X., Li, Z., Yue, W., Zhang, L. & Xie, Z. Toward at-home and wearable monitoring of female hormones: emerging nanotechnologies and clinical prospects. ACS Sens. (2025).
DiVasta, A. D. et al. Hormonal add-back therapy for females treated with gonadotropin-releasing hormone agonist for endometriosis. Obstet. Gynecol. 126, 617–627 (2015).
Google Scholar
Karakas, S. E. New biomarkers for diagnosis and management of polycystic ovary syndrome. Clin. Chim. Acta 471, 248–253 (2017).
Google Scholar
Constantin, A. Estradiol in systemic lupus erythematosus. Acta Endocrinol. (Buchar.) 19, 274–276 (2023).
Google Scholar
Xiang, D., Liu, Y., Zhou, S., Zhou, E. & Wang, Y. Protective effects of estrogen on cardiovascular disease mediated by oxidative stress. Oxid. Med. Cell Longev. 2021, 5523516 (2021).
Javed, A. et al. The relationship between myocardial infarction and estrogen use: a literature review. Cureus (2023).
Reisner, S. L. et al. Global health burden and needs of transgender populations: a review. Lancet 388, 412–436 (2016).
Google Scholar
Safer, J. D. et al. Barriers to healthcare for transgender individuals. Curr. Opin. Endocrinol. Diabetes Obes. 23, 168–171 (2016).
Google Scholar
Tangpricha, V. & den Heijer, M. Oestrogen and anti-androgen therapy for transgender women. Lancet Diabetes Endocrinol. 5, 291 (2016).
Google Scholar
Sudhakar, D., Huang, Z., Zietkowski, M., Powell, N. & Fisher, A. R. Feminizing gender-affirming hormone therapy for the transgender and gender diverse population: an overview of treatment modality, monitoring, and risks. Neurourol. Urodyn. 42, 903–920 (2023).
Google Scholar
Haimson, O. L., Gorrell, D., Starks, D. L. & Weinger, Z. Designing trans technology. In Proceedings of the 2020 CHI Conference on Human Factors in Computing Systems 1–13 (ACM, New York, NY, USA, 2020). https://doi.org/10.1145/3313831.3376669.
Johansson, T. et al. Contemporary menopausal hormone therapy and risk of cardiovascular disease: Swedish nationwide register based emulated target trial. BMJ e078784. (2024).
Kidd, J. D. et al. Prevalence of substance use and mental health problems among transgender and cisgender U.S. adults: Results from a national probability sample. Psychiatry Res. 326, 115339 (2023).
Google Scholar
Berliere, M. et al. Effects of hormones on breast development and breast cancer risk in transgender women. Cancers 15, 245 (2023).
Heng, Y. J. et al. Effect of testosterone therapy on breast tissue composition and mammographic breast density in trans masculine individuals. Breast Cancer Res. 26, 109 (2024).
Di Lisa, F. S. et al. Breast and cervical cancer in transgender men: literature review and a case report. Ther. Adv. Med. Oncol. 16, 17588359241259466 (2024).
Coelingh Bennink, H. J. T. et al. Progesterone from ovulatory menstrual cycles is an important cause of breast cancer. Breast Cancer Res. 25, 60 (2023).
Google Scholar
Chen, J.-H., Liu, H., Baek, H.-M., Nalcioglu, O. & Su, M.-Y. Magnetic resonance imaging features of fibrocystic change of the breast. Magn. Reson Imaging 26, 1207–1214 (2008).
Google Scholar
Zhu, Y. et al. Imaging manifestations of ductal adenoma of the breast: a case report. Open Life Sci. 19, 20220917 (2024).
Stachs, A., Stubert, J., Reimer, T. & Hartmann, S. Benign breast disease in women. Dtsch. Arztebl. Int. (2019).
McDonald, E. S., Clark, A. S., Tchou, J., Zhang, P. & Freedman, G. M. Clinical diagnosis and management of breast cancer. J. Nucl. Med. 57, 9S–16S (2016).
Google Scholar
Cardoso, F. et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 30, 1194–1220 (2019).
Google Scholar
Bleyer, A., Baines, C. & Miller, A. B. Impact of screening mammography on breast cancer mortality. Int. J. Cancer 138, 2003–2012 (2016).
Google Scholar
Novack, D. V. Estrogen and bone: osteoclasts take center stage. Cell Metab. 6, 254–256 (2007).
Google Scholar
Zhang, Y.-Y. et al. Insights and implications of sexual dimorphism in osteoporosis. Bone Res. 12, 8 (2024).
Google Scholar
Krugh, M. & Langaker, M. D. Dual-Energy X-Ray Absorptiometry. (2024).
Shah, B. A., Mirchandani, A. & Abrol, S. Impact of same day screening mammogram results on women’s satisfaction and overall breast cancer screening experience: a quality improvement survey analysis. BMC Women’s Health 22, 338 (2022).
Google Scholar
Ghomrawi, H. M. et al. Clinicians’ perspectives on wearable sensor technology as an alternative bedside monitoring tool in two West African countries. Int. J. Med. Inf. 175, 105046 (2023).
Google Scholar
Huhn, S. et al. The impact of wearable technologies in health research: scoping review. JMIR Mhealth Uhealth 10, e34384 (2022).
Google Scholar
Setyati, R. et al. The importance of early detection in disease management. J. World Future Med. 2, 51–63 (2024).
Spink, S. S. et al. High optode-density wearable diffuse optical probe for monitoring paced breathing hemodynamics in breast tissue. J. Biomed. Optics 26, 062708 (2021).
Mahmood, S. N. et al. Full ground ultra-wideband wearable textile antenna for breast cancer and wireless body area network applications. Micromachines 12, 322 (2021).
Google Scholar
Rahman, A., Islam, M. T., Singh, M. J., Kibria, S. & Akhtaruzzaman, M. Electromagnetic performances analysis of an ultra-wideband and flexible material antenna in microwave breast imaging: to implement a wearable medical bra. Sci. Rep. 6, 38906 (2016).
Google Scholar
Matiatou, M. et al. Complex refractive index of freshly excised human breast tissue as a marker of disease. Lasers Med. Sci. 37, 2597–2604 (2022).
Google Scholar
Du, W. et al. Conformable ultrasound breast patch for deep tissue scanning and imaging. Sci. Adv. 9, eadh5325 (2023).
Pandian, R., Danasegaran, S. K., Lalithakumari, S., Rajalakshmi, G. & Kumar, G. S. Photonic crystal based hour glass patch antenna for the detection of breast cancer. Opt. Quantum Electron 56, 1–11 (2024).
Google Scholar
Satpathy, S., Khalaf, O. I., Shukla, D. K., Algburi, S. & Hamam, H. Consumer electronics based smart technologies for enhanced terahertz healthcare having an integration of split learning with medical imaging. Sci. Rep. 14, 1–12 (2024).
Google Scholar
Elsheakh, D. M., Alsherif, S. A. & Eldamak, A. R. Textile monopole sensors for breast cancer detection. Telecommun. Syst. 82, 363–379 (2023).
Google Scholar
Elsheakh, D. N., Mohamed, R. A., Fahmy, O. M., Ezzat, K. & Eldamak, A. R. Complete breast cancer detection and monitoring system by using microwave textile based antenna sensors. Biosensors 13, 87 (2023).
Google Scholar
Vijayakumari, P. et al. Wearable transceiver with composite test-beds for breast cancer diagnosis. Mater. Today Proc. 45, 3120–3123 (2021).
Google Scholar
Mukai, Y. & Suh, M. Development of a conformal woven fabric antenna for wearable breast hyperthermia. Fash. Text. 8, 1–12 (2021).
Google Scholar
Elias, V., Rabih, A. & Nassar, G. Early breast lump detection using the intelligent bra”IN-bra”. HAL Open Sci. 4, 43–49 (2022).
Liu, L. & Webster, T. J. In situ sensor advancements for osteoporosis prevention, diagnosis, and treatment. Curr. Osteoporos. Rep. 14, 386–395 (2016).
Google Scholar
Amin, U., McPartland, A., O’Sullivan, M. & Silke, C. An overview of the management of osteoporosis in the aging female population. Women’s Health 19, 17455057231176655 (2023).
Kim, J. K., Bae, M. N., Lee, K., Kim, J. C. & Hong, S. G. Explainable artificial intelligence and wearable sensor-based gait analysis to identify patients with osteopenia and sarcopenia in daily life. Biosensors 12, 167 (2022).
Google Scholar
Kristoffersson, A. & Lindén, M. A systematic review on the use of wearable body sensors for health monitoring: a qualitative synthesis. Sensors 20, 1502 (2020).
Google Scholar
Aggelis, D. G. et al. Fracture of human femur tissue monitored by acoustic emission sensors. Sensors 15, 5803–5819 (2015).
Google Scholar
Song, Z., Wang, B., Zhang, Z., Yu, Y. & Lin, D. A highly flexible piezoelectric ultrasonic sensor for wearable bone density testing. Micromachines (Basel) 14, 1798 (2023).
Google Scholar
Gao, H. et al. Lower bone mineral density in patients with Parkinson’s disease: a cross-sectional study from Chinese Mainland. Front Aging Neurosci. 7, 162985 (2015).
Google Scholar
Stewart, A. & Black, A. Bone mineral density in osteoarthritis. Curr. Opin. Rheumatol. 12, 464–467 (2000).
Google Scholar
Lodder, M. C. et al. Bone mineral density in patients with rheumatoid arthritis: relation between disease severity and low bone mineral density. Ann. Rheum. Dis. 63, 1576–1580 (2004).
Google Scholar
Redelinghuys, M. J., Geldenhuys, J., Jung, H. & Kock, M. M. Bacterial vaginosis: current diagnostic avenues and future opportunities. Front Cell Infect. Microbiol. 10, 521070 (2020).
Google Scholar
Eleutério, J., Campaner, A. B. & de Carvalho, N. S. Diagnosis and treatment of infectious vaginitis: proposal for a new algorithm. Front Med. (Lausanne) 10, 1040072 (2023).
Google Scholar
Wilson, M. & Wilson, P. J. K. Vaginitis. Close Encounters of the Microbial Kind 361–378 (2021).
Rezk, S. & Alqabbasi, O. Bacterial vaginosis, vulvovaginal candidiasis, trichomonal vaginitis and aerobic vaginitis in women from Egypt. Germs 13, 130 (2023).
Google Scholar
Brown, H. & Drexler, M. Improving the diagnosis of vulvovaginitis: perspectives to align practice, guidelines, and awareness. Popul. Health Manag. 23, S3–S12 (2020).
Google Scholar
Jin, X. et al. An integrated and multi-target nucleic acid isothermal analysis system for rapid diagnosis of vulvovaginal candidiasis. Biosensors (Basel) 13, 559 (2023).
Google Scholar
Paghi, A. et al. Wireless and flexible optoelectronic system for in situ monitoring of vaginal pH using a bioresorbable fluorescence sensor. Adv. Mater. Technol. 8, 2201600 (2023).
Alma – Wearable Biosensor for Monitoring Vaginal Discharge – Hackster.io. https://www.hackster.io/alma/alma-wearable-biosensor-for-monitoring-vaginal-discharge-b1022f.
Pal, A., Nadiger, V. G., Goswami, D. & Martinez, R. V. Conformal, waterproof electronic decals for wireless monitoring of sweat and vaginal pH at the point-of-care. Biosens. Bioelectron. 160, 112206 (2020).
Torres, M. L. et al. Home-based electrochemical rapid sensor (HERS): a diagnostic tool for bacterial vaginosis. Sensors 23, 1891 (2023).
Google Scholar
Clack, K., Sallam, M., Matheson, C., Muyldermans, S. & Nguyen, N.-T. Towards a Wearable Feminine Hygiene Platform for Detection of Invasive Fungal Pathogens via Gold Nanoparticle Aggregation. Micromachines (Basel) 15, 899 (2024).
Vo, D.-K. & Trinh, K. T. L. Advances in wearable biosensors for healthcare: current trends, applications, and future perspectives. Biosensors (Basel) 14, 560 (2024).
Google Scholar
World Health Organization. Point-of-Care Diagnostic Tests (POCTs) for Sexually Transmitted Infections (STIs). World Health Organization https://www.who.int/teams/sexual-and-reproductive-health-and-research-(srh)/areas-of-work/sexual-health/sexually-transmitted-infections/point-of-care-tests#:~:text=Background%20and%20rationale,professional%20and%20lay%20health%20workers.
Adamson, P. C., Loeffelholz, M. J. & Klausner, J. D. Point-of-care testing for sexually transmitted infections a review of recent developments. Arch. Pathol. Lab. Med. 144, 1344 (2020).
Google Scholar
Chen, L., Li, J. & Xiao, B. The role of sialidases in the pathogenesis of bacterial vaginosis and their use as a promising pharmacological target in bacterial vaginosis. Front. Cell. Infection Microbiol. 14, 1367233 (2024).
Wu, S. et al. A biochemiluminescent sialidase assay for diagnosis of bacterial vaginosis. Sci. Rep. 9, 20024 (2019).
Rodríguez-Nava, C. et al. Nanophotonic sialidase immunoassay for bacterial vaginosis diagnosis. ACS Pharm. Transl. Sci. 4, 365–371 (2021).
Google Scholar
Ng, S. et al. Large-scale characterisation of the pregnancy vaginal microbiome and sialidase activity in a low-risk Chinese population. NPJ Biofilms Microbiomes 7, 89 (2021).
Zhang, Y. & Rochefort, D. Fast and effective paper based sensor for self-diagnosis of bacterial vaginosis. Anal. Chim. Acta 800, 87–94 (2013).
Google Scholar
Reukov, V. et al. Fabrication of nanocoated fibers for self-diagnosis of bacterial vaginosis. Mater. Sci. Eng. C 29, 669–673 (2009).
Google Scholar
Avila-Huerta, M. D. et al. Disposable device for bacterial vaginosis detection. ACS Meas. Sci. Au. 3, 355–360 (2023).
Google Scholar
Li, M. et al. Loop-mediated isothermal amplification (LAMP): potential point-of-care testing for vulvovaginal candidiasis. J. Fungi 9, 1159 (2023).
Mosley, G. L. et al. Improved lateral-flow immunoassays for chlamydia and immunoglobulin M by sequential rehydration of two-phase system components within a paper-based diagnostic. Microchim. Acta 184, 4055–4064 (2017).
Google Scholar
Yang, J. et al. Leak-proof probe for accurate detection of Neisseria gonorrhoeae by recombinase polymerase amplification-mediated lateral flow strip. Anal. Chim. Acta 1258, 341176 (2023).
Ji, T. et al. Establishment and application of a rapid visual diagnostic method for Streptococcus agalactiae based on recombinase polymerase amplification and lateral flow strips. Sci. Rep. 14, 10064 (2024).
Dighe, K. et al. Highly-specific single-stranded oligonucleotides and functional nanoprobes for clinical determination of chlamydia trachomatis and neisseria gonorrhoeae infections. Adv. Sci. 10, 2304009 (2023).
Chen, X. et al. Nanoparticle-based lateral flow biosensor integrated with loop-mediated isothermal amplification for rapid and visual identification of chlamydia trachomatis for point-of-care use. Front. Microbiol. 13, 914620 (2022).
Google Scholar
Chen, X. et al. Visual and rapid identification of Chlamydia trachomatis and Neisseria gonorrhoeae using multiplex loop-mediated isothermal amplification and a gold nanoparticle-based lateral flow biosensor. Front Cell Infect. Microbiol. 13, 1067554 (2023).
Google Scholar
Shivaram, K. B., Bhatt, P., Verma, M. S., Clase, K. & Simsek, H. Bacteriophage-based biosensors for detection of pathogenic microbes in wastewater. Sci. Total Environ. 901, 165859 (2023).
Patel, D., Zhou, Y. & Ramasamy, R. P. A bacteriophage-based electrochemical biosensor for detection of methicillin-resistant staphylococcus aureus. J. Electrochem. Soc. 168, 057523 (2021).
Google Scholar
Panhwar, S., Keerio, H. A., Ilhan, H., Boyacı, I. H. & Tamer, U. Principles, methods, and real-time applications of bacteriophage-based pathogen detection. Mol. Biotechnol. 2023 1–18 (2023).
Wu, L. et al. Multiplexed detection of bacterial pathogens based on a cocktail of dual-modified phages. Anal. Chim. Acta 1166, 338596 (2021).
Google Scholar
Fitzgerald, R. C., Antoniou, A. C., Fruk, L. & Rosenfeld, N. The future of early cancer detection. Nat. Med. 28, 666–677 (2022).
Guerra, C. E., Sharma, P. V. & Castillo, B. S. Annual Review of Medicine Multi-Cancer Early Detection: The New Frontier in Cancer Early Detection. (2023).
Pink, R. C., Beaman, E. M., Samuel, P., Brooks, S. A. & Carter, D. R. F. Utilising extracellular vesicles for early cancer diagnostics: benefits, challenges and recommendations for the future. Br J Cancer 126, 323–330 (2022).
Aziz, N. B. et al. MicroRNAs in ovarian cancer and recent advances in the development of microRNA-based biosensors. Analyst 145, 2038–2057 (2020).
Giampaolino, P. et al. Role of biomarkers for early detection of ovarian cancer recurrence. Gland Surg. 9, 1102–1111 (2020).
Trinidad, C. V., Tetlow, A. L., Bantis, L. E. & Godwin, A. K. Reducing ovarian cancer mortality through early detection: approaches using circulating biomarkers. Cancer Prev. Res. 13, 241–252 (2020).
Lone, S. N. et al. Liquid biopsy: a step closer to transform diagnosis, prognosis and future of cancer treatments. Mol. Cancer 21, 79 (2022).
Belotti, Y. & Lim, C. T. Microfluidics for liquid biopsies: recent advances, current challenges, and future directions. Anal. Chem. 93, 4727–4738 (2021).
Lin, B. et al. Microfluidic-based exosome analysis for liquid biopsy. Small Methods 5, 2001131 (2021).
Poudineh, M. et al. Tracking the dynamics of circulating tumour cell phenotypes using nanoparticle-mediated magnetic ranking. Nat. Nanotechnol. 12, 274–281 (2017).
Google Scholar
Poudineh, M., Sargent, E. H., Pantel, K. & Kelley, S. O. Profiling circulating tumour cells and other biomarkers of invasive cancers. Nat. Biomed. Eng. 2, 72–84 (2018).
Gardner, L., Kostarelos, K., Mallick, P., Dive, C. & Hadjidemetriou, M. Nano-omics: nanotechnology-based multidimensional harvesting of the blood-circulating cancerome. Nat. Rev. Clin. Oncol. 19, 551–561 (2022).
Google Scholar
Zhao, Z., Yang, Y., Zeng, Y. & He, M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip 16, 489–496 (2016).
Google Scholar
Sinawang, P. D., Soto, F., Ozen, M. O., Akin, D. & Demirci, U. Progress and challenges in biomarker enrichment for cancer early detection. Progr. Biomed. Eng. 3, 043001 (2021).
Labib, M. et al. Tracking the expression of therapeutic protein targets in rare cells by antibody-mediated nanoparticle labelling and magnetic sorting. Nat. Biomed. Eng. 5, 41–52 (2021).
Google Scholar
Li, F., Yang, G., Aguilar, Z. P., Xiong, Y. & Xu, H. Affordable and simple method for separating and detecting ovarian cancer circulating tumor cells using BSA coated magnetic nanoprobes modified with folic acid. Sens Actuators B Chem. 262, 611–618 (2018).
Google Scholar
Ma, J. et al. Purification of circulating tumor cells based on multiantibody-modified magnetic nanoparticles and molecular analysis toward epithelial ovarian cancer detection. ACS Sens. 8, 3744–3753 (2023).
Google Scholar
Gurudatt, N. G. et al. Separation detection of different circulating tumor cells in the blood using an electrochemical microfluidic channel modified with a lipid-bonded conducting polymer. Biosens. Bioelectron. 146, 111746 (2019).
Descamps, L., Le Roy, D. & Deman, A. L. Microfluidic-based technologies for CTC isolation: a review of 10 years of intense efforts towards liquid biopsy. Int. J. Mol. Sci. 23, 1981 (2022).
Karabacak, N. M. et al. Microfluidic, marker-free isolation of circulating tumor cells from blood samples. Nat. Protoc. 9, 694–710 (2014).
Google Scholar
Fang, S. et al. Clinical application of a microfluidic chip for immunocapture and quantification of circulating exosomes to assist breast cancer diagnosis and molecular classification. PLoS One 12, e0175050 (2017).
Gwak, H. et al. Microfluidic chip for rapid and selective isolation of tumor-derived extracellular vesicles for early diagnosis and metastatic risk evaluation of breast cancer. Biosens. Bioelectron. 192, 113495 (2021).
Chen, W. et al. Simple and fast isolation of circulating exosomes with a chitosan modified shuttle flow microchip for breast cancer diagnosis. Lab Chip 21, 1759–1770 (2021).
Google Scholar
Gumus, E., Bingol, H. & Zor, E. Lateral flow assays for detection of disease biomarkers. J. Pharm. Biomed. Anal. 225, 115206 (2023).
Ren, W., Mohammed, S. I., Wereley, S. & Irudayaraj, J. Magnetic focus lateral flow sensor for detection of cervical cancer biomarkers. Anal. Chem. 91, 2876–2884 (2019).
Google Scholar
Ekman, M. et al. Spectrally separated dual-label upconversion luminescence lateral flow assay for cancer-specific STn-glycosylation in CA125 and CA15-3. Anal. Bioanal. Chem. 13, 3251–3260 (2024).
Chen, J. et al. Accurate and portable tumor exosomes detection based on manganese dioxide and aptamer-functionalized fluorescent microspheres mediated dual-mode lateral flow assay. Sens. Actuators B Chem. 409, 135614 (2024).
Zhang, B. et al. Fluorescence quenching-based signal amplification on immunochromatography test strips for dual-mode sensing of two biomarkers of breast cancer. Nanoscale 9, 18711–18722 (2017).
Google Scholar
Guo, S. et al. All-in-one detection of breast cancer-derived exosomal miRNA on a pen-based paper chip. Analyst 149, 1250–1261 (2024).
Google Scholar
Liu, H., Cao, J. & Ding, S. N. Simultaneous detection of two ovarian cancer biomarkers in human serums with biotin-enriched dendritic mesoporous silica nanoparticles-labeled multiplex lateral flow immunoassay. Sens. Actuators B Chem. 371, 132597 (2022).
Szymanska, B., Lukaszewski, Z., Hermanowicz-Szamatowicz, K. & Gorodkiewicz, E. A Multiple-array SPRi biosensor as a tool for detection of gynecological–oncological diseases. Biosensors (Basel) 13, 279 (2023).
Google Scholar
Kight, E. C., Hussain, I., Bowden, A. K. & Haselton, F. R. Recurrence monitoring for ovarian cancer using a cell phone-integrated paper device to measure the ovarian cancer biomarker HE4/CRE ratio in urine. Sci. Rep. 11, 21945 (2021).
Google Scholar
Tellez-Gabriel, M., Knutsen, E. & Perander, M. Current status of circulating tumor cells, circulating tumor DNA, and exosomes in breast cancer liquid biopsies. Int. J. Mol. Sci. 21, 1–23 (2020).
Jordan, N. V. et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature 537, 102–106 (2016).
Google Scholar
Meng, S. et al. Surface-enhanced Raman scattering holography chip for rapid, sensitive and multiplexed detection of human breast cancer-associated MicroRNAs in clinical samples. Biosens. Bioelectron. 190, 113470 (2021).
Yoon, J. et al. Flexible electrochemical biosensors for healthcare monitoring. J. Mater. Chem. B 8, 7303–7318 (2020).
Kucherenko, I. S., Soldatkin, O. O., Kucherenko, D. Y., Soldatkina, O. V. & Dzyadevych, S. V. Advances in nanomaterial application in enzyme-based electrochemical biosensors: a review. Nanoscale Adv. 1, 4560–4577 (2019).
Tang, Y., Liu, Y., Xia, Y., Zhao, F. & Zeng, B. Simultaneous detection of ovarian cancer-concerned HE4 and CA125 markers based on Cu single-atom-triggered CdS QDs and Eu MOF@Isoluminol ECL. Anal. Chem. 95, 4795–4802 (2023).
Google Scholar
Barr, C. E., Njoku, K., Owens, G. L. & Crosbie, E. J. Urine CA125 and HE4 for the detection of ovarian cancer in symptomatic women. Cancers (Basel) 15, 1256 (2023).
Google Scholar
Ross, G. M. S., Filippini, D., Nielen, M. W. F. & Salentijn, G. I. J. Unraveling the Hook effect: a comprehensive study of high antigen concentration effects in sandwich lateral flow immunoassays. Anal. Chem. 92, 15587–15595 (2020).
Google Scholar
Su, X. et al. Integrated SERS-vertical flow biosensor enabling multiplexed quantitative profiling of serological exosomal proteins in patients for accurate breast cancer subtyping. ACS Nano 17, 4077–4088 (2023).
Google Scholar
Warty, R. R., Smith, V., Patabendige, M., Fox, D. & Mol, B. Clarifying the unmet clinical need during medical device innovation in women’s health—a narrative review. Health Care Women Int. 45, 1–29 (2023).
Regidor, P. A., Kaczmarczyk, M., Schiweck, E., Goeckenjan-Festag, M. & Alexander, H. Identification and prediction of the fertile window with a new web-based medical device using a vaginal biosensor for measuring the circadian and circamensual core body temperature. Gynecol. Endocrinol. 34, 256–260 (2018).
Google Scholar
Zhu, T. Y. et al. The accuracy of wrist skin temperature in detecting ovulation compared to basal body temperature: prospective comparative diagnostic accuracy study. J. Med. Internet Res. 23, e20710 (2021).
Google Scholar
Hicks, J. L. et al. Best practices for analyzing large-scale health data from wearables and smartphone apps. npj Digit. Med. 2, 45 (2019).
Walter, J. R., Xu, S. & Rogers, J. A. From lab to life: how wearable devices can improve health equity. Nat. Commun. 15, 123 (2024).
Mihan, A. & Van Spall, H. G. C. Interventions to enhance digital health equity in cardiovascular care. Nat. Med. 30, 628–630 (2024).
Dexcom. Manage your diabetes with confidence. https://www.dexcom.com/en-CA.
FreeStyle. What is FreeStyle Libre? https://www.freestyle.abbott/en-ca/products/what-is-free-style-libre.html?utm_source=google&utm_medium=cpc&utm_campaign=FSL_Brand&utm_content=106128906887&utm_term=freestyle%20libre&gad_source=1&gad_campaignid=10263421839&gbraid=0AAAAADbRZUNt21gagBE8nuAAfu9mqYO2N&gclid=CjwKCAjw6NrBBhB6EiwAvnT_ruv0vdiuV0kBMFf1LsZoi-AhRfDc7RXwy5nUtJTzE-c6iMCJaZ-DGxoCNIkQAvD_BwE.
Doshi, K. B., Moon, S. H., Whitaker, M. D. & Lockhart, T. E. Assessment of gait and posture characteristics using a smartphone wearable system for persons with osteoporosis with and without falls. Sci. Rep. 13, 1–9 (2023).
Google Scholar
Huberty, J., Ehlers, D. K., Kurka, J., Ainsworth, B. & Buman, M. Feasibility of three wearable sensors for 24 hour monitoring in middle-aged women. BMC Women’s Health 15, 1–9 (2015).
Google Scholar
Sanchez-Trigo, H., Maher, C., Godino, J. G. & Sañudo, B. Effects of an mHealth physical activity intervention to prevent osteoporosis in premenopausal women. A randomized controlled trial. J. Sci. Med. Sport 26, 545–552 (2023).
Google Scholar
Sánchez-Trigo, H., Sanchez-Oliver, A. J., Abt, G. & Sañudo, B. Validation of a Wearable accelerometer-based activity monitor for use in future osteoporosis prevention programs. Sustainability 12, 2187 (2020).
Google Scholar
Navalta, J. W., Ramirez, G. G., Maxwell, C., Radzak, K. N. & McGinnis, G. R. Validity and reliability of three commercially available smart sports bras during treadmill walking and running. Sci. Rep. 10, 1–9 (2020).
Google Scholar
Arcarisi, L. et al. Palpreast—a new wearable device for breast self-examination. Appl. Sci. 9, 381 (2019).
Google Scholar
Park, C. K. S. et al. Cost-effective, portable, patient-dedicated three-dimensional automated breast ultrasound for point-of-care breast cancer screening. Sci. Rep. 13, 1–16 (2023).
Broach, R. B. et al. A cost-effective handheld breast scanner for use in low-resource environments: a validation study. World J. Surg. Oncol. 14, 1–6 (2016).
Google Scholar
Fertility Test Market by Product (Ovulation Predictor Kits, Fertility Monitors (Urine, Saliva, Blood)), Mode of Purchase (OTC, Prescription, Online), Application (Female, Male), End User (Home care, Fertility clinics, hospitals) & Region – Global Forecast to 2025. https://www.marketsandmarkets.com/Market-Reports/fertility-testing-devices-market-139945432.html#:~:text=Market%20Growth%20Outlook%20Summary,both%20developed%20and%20developing%20countries.
Gestational Diabetes Market Size And Share Analysis – Growth Trends And Forecasts (2025-2032). https://www.coherentmi.com/industry-reports/gestational-diabetes-market.
Patenting products for women. https://www.taylorwessing.com/en/insights-and-events/insights/2024/06/patenting-products-for-women.
Mshelia, Y. U., Elei, F. O., Ikerionwu, C. O., Erike, A. I. & Mshelia, Y. U. Transition to Value-Based Care IoT Wearable Devices in Maternity Healthcare for Low-Resource Countries. Int. J. Sci. Res. Comput. Sci. Eng. 11, 8–14 (2023).
Google Scholar
Godfrey, A. et al. Wearables beyond borders: a case study of barriers to gait assessment in low-resource settings. Maturitas 137, 7–10 (2020).
Google Scholar
Paolillo, E. W. et al. Wearable use in an observational study among older adults: adherence, feasibility, and effects of clinicodemographic factors. Front Digit Health 4, 884208 (2022).
Google Scholar
Li, J., Ma, Q., Chan, A. H. & Man, S. S. Health monitoring through wearable technologies for older adults: smart wearables acceptance model. Appl. Erg. 75, 162–169 (2019).
Google Scholar
Tikkanen, H., Heinonen, K. & Ravald, A. Smart wearable technologies as resources for consumer agency in well-being. J. Interact. Mark. 58, 136–150 (2023).
Google Scholar
Yao, R. et al. Inequities in health care services caused by the adoption of digital health technologies: scoping review. J. Med. Internet Res. 24, e34144 (2022).
Google Scholar
Biga, R., Nottebaum, S., Kozlakidis, Z. & Psomiadis, S. Digitalization of Healthcare in LMICs: Digital Health and the Digital Divide Based on Technological Availability and Development. In 185–193. (2024).
Borges do Nascimento, I. J. et al. Transforming women’s health, empowerment, and gender equality with digital health: evidence-based policy and practice. Lancet Digit. Health (2025).
Berrian, K., Exsted, M. D., Lampe, N. M., Pease, S. L. & Akré, E. L. Barriers to quality healthcare among transgender and gender nonconforming adults. Health Serv. Res. 60, e14362 (2025).
Renner, J. et al. Barriers to accessing health care in rural regions by transgender, non-binary, and gender diverse people: a case-based scoping review. Front. Endocrinol. (Lausanne) 12, 717821 (2021).
Safer, J. D. & Chan, K. J. Review of medical, socioeconomic, and systemic barriers to transgender care. In 25–38. (2019).
Cook, R. Women’s Health and Human Rights The Promotion and Protection of Women’s Health through International Human Rights law. World Health Organization Geneva. (1994).
Women’s autonomy, equality and reproductive health | OHCHR. https://www.ohchr.org/en/special-procedures/wg-women-and-girls/womens-autonomy-equality-and-reproductive-health.
2024 – Women’s Health Manifesto – Eurohealth. https://eurohealth.ie/2023/05/29/2024-womens_health_manifesto/.
Daniel, H. et al. Women’s health policy in the united states: an american college of physicians position paper. Ann. Intern Med. 168, 874–875 (2018).
Google Scholar
Women’s health and well-being in Europe: beyond the mortality advantage. (2016).
Women’s Health Initiative (WHI) | NHLBI, NIH. https://www.nhlbi.nih.gov/science/womens-health-initiative-whi.
National Women’s Health Strategy (2020-2030) – Australian Women’s Health Alliance. https://australianwomenshealth.org/resource/national-womens-health-strategy/.
United Nations. United Nations Population Fund. United Nations. https://www.unfpa.org/donate/EmergencyBirthKit/b?utm_source=google&utm_medium=cpc&utm_campaign=UNFPA_DLV_GAdsP_Search_Brand_Alpha_Tier1_EN&utm_content=Evergreen&gad_source=1&gad_campaignid=22480363807&gbraid=0AAAAAoaU5jLKIgEPYbRPuycqhN2wHuvDE&gclid=EAIaIQobChMI3L35tt26jwMVW1N_AB3W6TrWEAAYASAAEgKmavD_BwE.
World Health Organization. Global Strategy for Women’s, Children’s and Adolescents’ Health Data Portal. World Health Organization. https://platform.who.int/data/maternal-newborn-child-adolescent-ageing/global-strategy-data.
Ye, J., Hai, J., Song, J. & Wang, Z. The role of artificial intelligence in the application of the integrated electronic health records and patient-generated health data. medRxiv 2024.05.01.24306690 (2024).
Wang, Z. et al. AI-assisted diagnosis of vulvovaginal candidiasis using cascaded neural networks. Microbiol. Spectr. 13, e01691–24 (2025).
Mennickent, D. et al. Machine learning applied in maternal and fetal health: a narrative review focused on pregnancy diseases and complications. Front. Endocrinol. (Lausanne) 14, 1130139 (2023).
Zhang, Z. et al. Machine learning prediction models for gestational diabetes mellitus: meta-analysis. J. Med. Internet Res. 24, e26634 (2022).
Google Scholar
Foschi, C. et al. Potential use of artificial intelligence for vaginal swab analysis in the assessment of common genital disorders: a pilot study. New Microbiol. 45, 291 (2022).
Zhu, X. et al. Cervical cancer screening aided by artificial intelligence, China. Bull. World Health Organ. 101, 381 (2023).
Google Scholar
McKinney, S. M. et al. International evaluation of an AI system for breast cancer screening. Nature 577, 89–94 (2020).
Google Scholar
Ng, A. Y. et al. Prospective implementation of AI-assisted screen reading to improve early detection of breast cancer. Nat. Med. 29, 3044–3049 (2023).
Google Scholar
Salim, M. et al. AI-based selection of individuals for supplemental MRI in population-based breast cancer screening: the randomized ScreenTrustMRI trial. Nat. Med. (2024).
van Dooijeweert, C. et al. Clinical implementation of artificial-intelligence-assisted detection of breast cancer metastases in sentinel lymph nodes: the CONFIDENT-B single-center, non-randomized clinical trial. Nat. Cancer 5, 1195–1205 (2024).
Google Scholar
Lotter, W. et al. Robust breast cancer detection in mammography and digital breast tomosynthesis using an annotation-efficient deep learning approach. Nat. Med. 27, 244–249 (2021).
Google Scholar
Burks, J. H. et al. General feature selection technique supporting sex-debiasing in chronic illness algorithms validated using wearable device data. npj Women’s Health 2, 37 (2024).
Google Scholar
Simms, K. T. et al. Benefits, harms and cost-effectiveness of cervical screening, triage and treatment strategies for women in the general population. Nat. Med. 29, 3050–3058 (2023).
Google Scholar
Endo, P. T. Artificial intelligence for women and child healthcare: is AI able to change the beginning of a new story? A perspective. Health Sci. Rep. 8, e70779 (2025).
Massachusetts General Hospital. Are you pregnant? Do you own a smartphone? MGH’s Center for Women’s Mental Health. (2025).
Pearlstein, T., Howard, M., Salisbury, A. & Zlotnick, C. Postpartum depression. Am. J. Obstet. Gynecol. 200, 357–364 (2009).
Google Scholar
Davidson, L. & Boland, M. R. Towards deep phenotyping pregnancy: a systematic review on artificial intelligence and machine learning methods to improve pregnancy outcomes. Brief Bioinform. 22, bbaa369 (2021).
OvuFirst Wearable Fertility Tracker. https://www.hellovio.com/en_ca/about-ovufirst/.
GE HealthCare (United States). Invenia Abus Breast Imaging Ultrasound. https://www.gehealthcare.com/products/ultrasound/breast-ultrasound/invenia-abus.
Futoma, J., Simons, M., Panch, T., Doshi-Velez, F. & Celi, L. A. The myth of generalisability in clinical research and machine learning in health care. Lancet Digit Health 2, e489–e492 (2020).
Google Scholar
Achtari, M. et al. Gender bias in AI’s perception of cardiovascular risk. J. Med. Internet Res. 26, e54242 (2024).
Google Scholar
Bruce, L. K. et al. Variability of temperature measurements recorded by a wearable device by biological sex. Biol. Sex. Differ. 14, 76 (2023).
Google Scholar
Smarr, B. L. AI for precision medicine must keep non-random complexity in mind to support equity in outcomes. In 2024 IEEE 20th International Conference on e-Science (e-Science) 1–7 (IEEE, 2024). https://doi.org/10.1109/e-Science62913.2024.10678664.
Cirillo, D. et al. Sex and gender differences and biases in artificial intelligence for biomedicine and healthcare. NPJ Digit Med. 3, 81 (2020).
Google Scholar
Murrin, E. M., Saad, A. F., Sullivan, S., Millo, Y. & Miodovnik, M. Innovations in diabetes management for pregnant women: artificial intelligence and the internet of medical things. Am. J. Perinatol. (2024).
Abu-El-Ruz, R. et al. Artificial intelligence in bacterial infections control: a scoping review. Antibiotics 14, 256 (2025).
Google Scholar
link